The van Halem Group Quarterly Newsletter - Q2 2024
Compliance Mastery in Healthcare
In the complex landscape of healthcare, compliance is a critical aspect that healthcare providers must navigate with precision and care. The Office of Inspector General (OIG) plays a pivotal role in setting the standards for compliance, offering guidance to ensure providers adhere to healthcare regulations and requirements. The OIG has created a series of documents for many segments in the healthcare industry to include durable medical equipment suppliers. These documents are often referred to as compliance program guidance documents which allow suppliers to develop and use internal controls that monitor regulations and other program requirements.
- Main Components of OIG Compliance
- Voluntary Compliance Program
- Education and Training
- Advisory Opinions
- Compliance Resources
Healthcare providers play an essential role in securing compliance with OIG regulations. This can be done by engaging the OIG’s guidance with a robust compliance program tailored to their specific operations, in addition to staying in tune with changes and updates to compliance requirements.
Compliance with healthcare regulations and requirements is a moral obligation in addition to a legal one. As the HME industry continues to grow, compliance will remain a cornerstone of its success by embracing regulatory standards and implementing compliance programs.
For example, the OIG’s announcement in February 2024, explained OIG anticipates that the first two industry segment-specific CPGs (ICPGs) will address Medicare Advantage and nursing facilities. OIG intends to publish these guidance documents in 2024.
As we continue into the third quarter of 2024, The van Halem Group continues to support our compliance clients as we assist with regulatory issues, multi-level audit and appeal support, and many other complex issues.
Stories of Fraud and Abuse
Acts
Support H.R. 5555-DMEPOS Relief Act of 2023
Home Medical Equipment suppliers are running on empty. Supply chain disruptions, product recalls, increased fuel prices, staffing issues, and employee salary increases, have exacerbated the reimbursement strain suppliers experience. New legislation was introduced by Reps. Mariannette Miller-Meeks (R-IA) and Paul Tonko (D-NY). H.R. 5555, The DMEPOS Relief Act of 2023, provides a 90/10 blended Medicare reimbursement rate (90% adjusted payment rate/10% unadjusted fee schedule rate) for most home medical equipment products in Competitive Bidding Areas (CBAs), effective Jan. 1, 2024, through Dec. 31, 2024. There is also language in section 3 of the legislation to extend the current 75/25 blended rate in effect for non-rural/non-CBA suppliers through 2024. Help secure co-sponsors for legislation to provide reimbursement relief for former Competitive Bidding Areas!
Support S. 1294-75/25 Blended Rate
This blended rate was introduced to counter the decline in reimbursement rates experienced in the years leading up to the CARES Act. To maintain the benefits of the 75/25 blended rate, industry stakeholders alike have rallied behind Senate bill S. 1294. This bill aims to extend the blended rate for non-rural areas until the end of the 2024.
Compliance Tip
Comprehending updates to Corporate Integrity Agreements (CIAs) equips compliance officers with key focal points for enhancing compliance initiatives and performing risk evaluations. Engaging industry compliance experts to review the effectiveness of an existing compliance program can provide invaluable insights into areas that may require improvement or adjustment.
Audit Corner
Audit Alerts - SMRC
As the Supplemental Medical Review Contractor (SMRC), Noridian conducts nationwide medical reviews (Part A, Part B, and DME), in accordance with all applicable statutes, laws, regulations, national and local coverage determination policies, and coding guidance, to determine whether Medicare claims have been billed in compliance with coverage, coding, payment, and billing practices. For more information, visit the SMRC at: https://www.noridiansmrc.com/.

At CMS discretion, not all projects will be made available on the SMRC website.
Audit Alerts - DME MACs
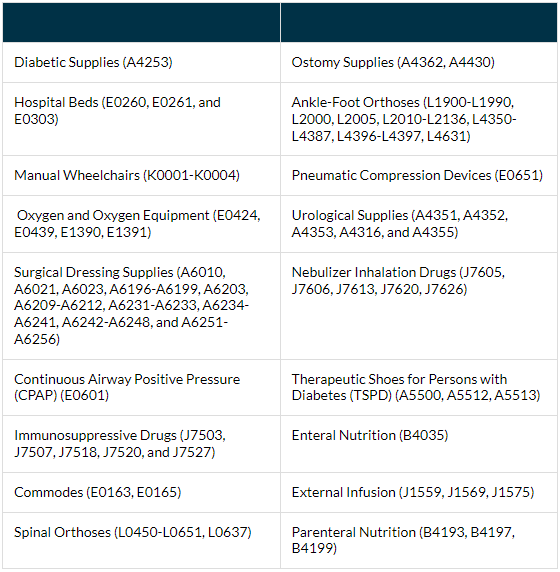
DME MACs conduct Targeted Probe and Educate (TPE) prepayment audits to review DMEPOS claims. The chart below is a compiled list of items currently under TPE review for all four DME MAC Jurisdictions.
Audit Alerts – OIG
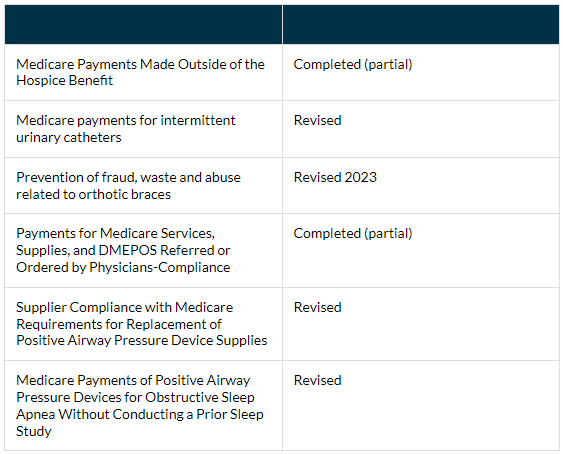
The Office of Inspector General (OIG) Work Plan sets forth various projects including audits and evaluations that are underway or planned to be addressed during the fiscal year and beyond. The OIG updates their plan monthly, to the Recently Added Items page. The OIG Work Plan is a good indicator of the types of equipment and services that may be reviewed by other audit contractors such as the UPIC, Supplemental Medical Review Contractor, Medicare Administrative Contractor, and Comprehensive Error Rate Testing. The following DMEPOS items are currently open items on the Work Plan:
Audit Alerts- RAC
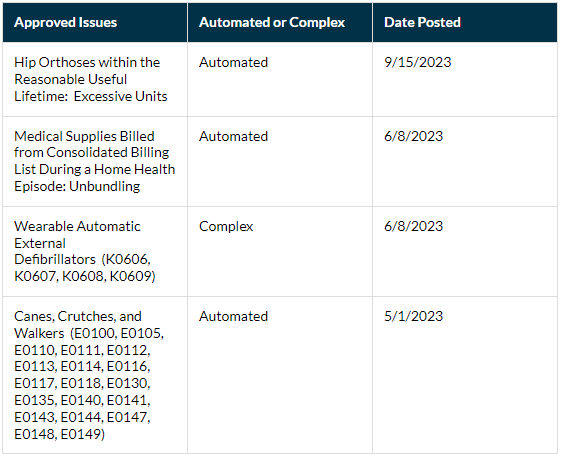
Performant Recovery, the National Home Health and Hospice and DMEPOS Recovery Audit Contractor (RAC) can only audit claims having a “claim paid date” which is less than or equal to three years prior to the Demand Letter date. Claims having a paid date over three years are excluded from audit. CMS approved issues are posted to Performant’s website here. Below are the most recently posted issues; however, all issues posted to the website are susceptible to audit unless marked as closed.
Upcoming Events
For a complete listing of all vHG events, information, and to register, visit the Event Calendar on our website here.
Are You in the Know?
If you are not subscribed to The van Halem Group Blog, then you could be missing out on valuable industry information!
Any additional compliance information you would like to see in our newsletter? We love to hear your input!


